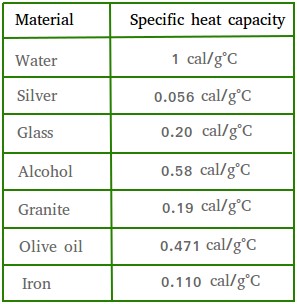
SOLVED: The specific heat capacity of iron is 0.45 J/g °C. How many joules of energy are needed to warm 1.96 g of iron from 20.00 °C to 29.0 °C? Determine the

A piece of iron of mass 100g is kept inside a furnace for a long time and then put in a calorimeter of water equivalent 10g containing 240g of water at 20^oC .

Figure 3 from Thermodynamics of iron sulfides I. Heat capacity and thermodynamic properties of Fe9S10 at temperatures from 5 K to 740 K | Semantic Scholar
A 1.22-kg piece of iron at 126.5 °C is dropped into 981 g water at 22.1 °C. The temperature rises to 34.4 °C, what is the specific heat of iron, in J g-1°C-1? - Quora

Calculate the amount of heat required to raise the temperature of 5 g of iron from `25^(@)C \"to\" - YouTube

Heat Capacity of Iron Lab - Heat Capacity of Iron Lab Report Samantha Himegarner Chemistry 4A Purpose The purpose of this lab is to determine the | Course Hero
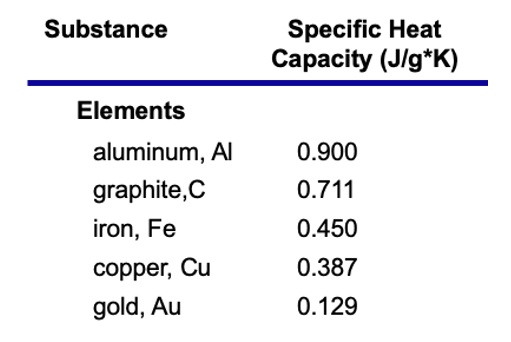
SOLVED: Text: Substance Specific Heat Capacity (J/g*K) Elements: aluminum, Al; graphite, C; iron, Fe; copper, Cu; gold, Au 0.900 J/g*K, 0.711 J/g*K, 0.450 J/g*K, 0.387 J/g*K, 0.129 J/g*K




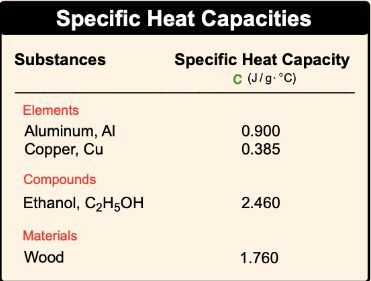




![Molar heat capacity of iron. Symbols indicate measurements from [10]. | Download Scientific Diagram Molar heat capacity of iron. Symbols indicate measurements from [10]. | Download Scientific Diagram](https://www.researchgate.net/publication/332219094/figure/fig1/AS:744154090987520@1554431569138/Molar-heat-capacity-of-iron-Symbols-indicate-measurements-from-10.png)