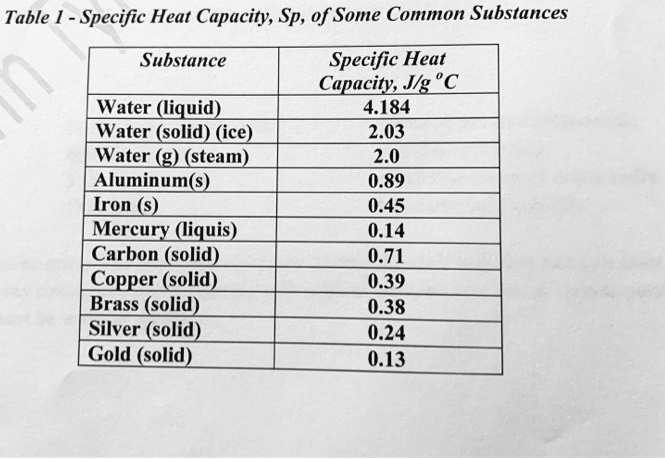
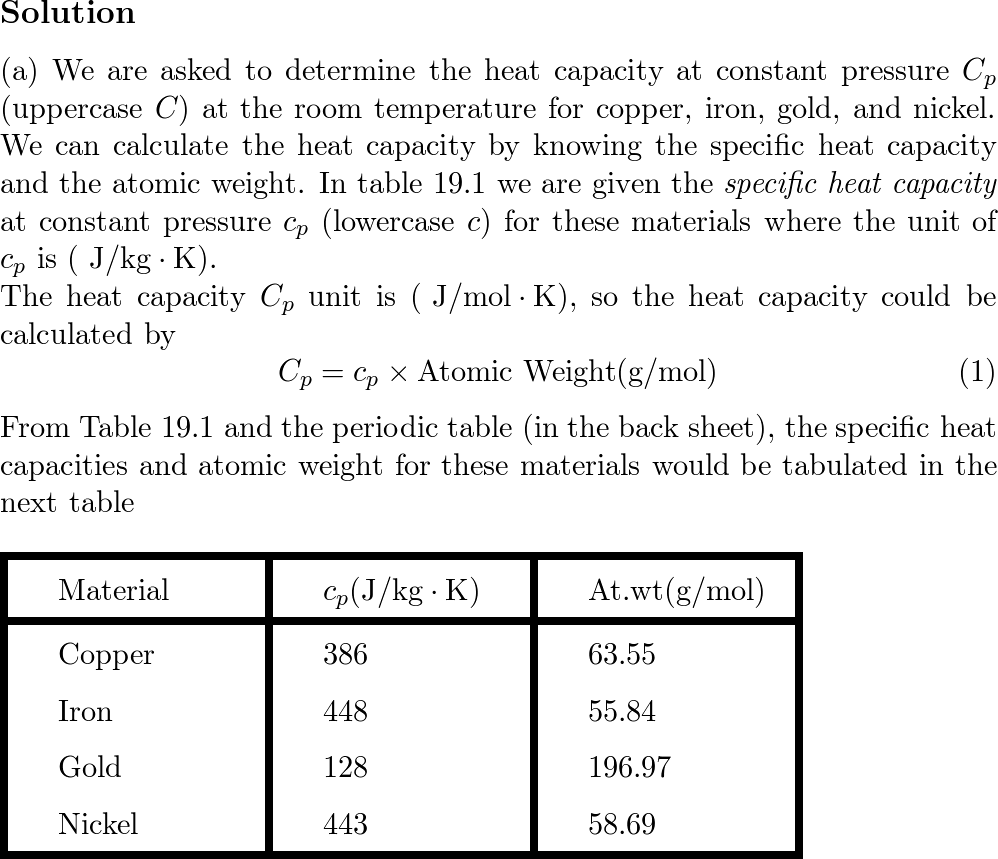
SOLVED: Table 1: Specific Heat Capacity, Sp, of Some Common Substances Substance Specific Heat Capacity, J/g 4.184 2.03 2.0 0.89 0.45 0.14 0.71 0.39 0.38 0.24 0.13 Water (liquid) Water (solid) (ice)
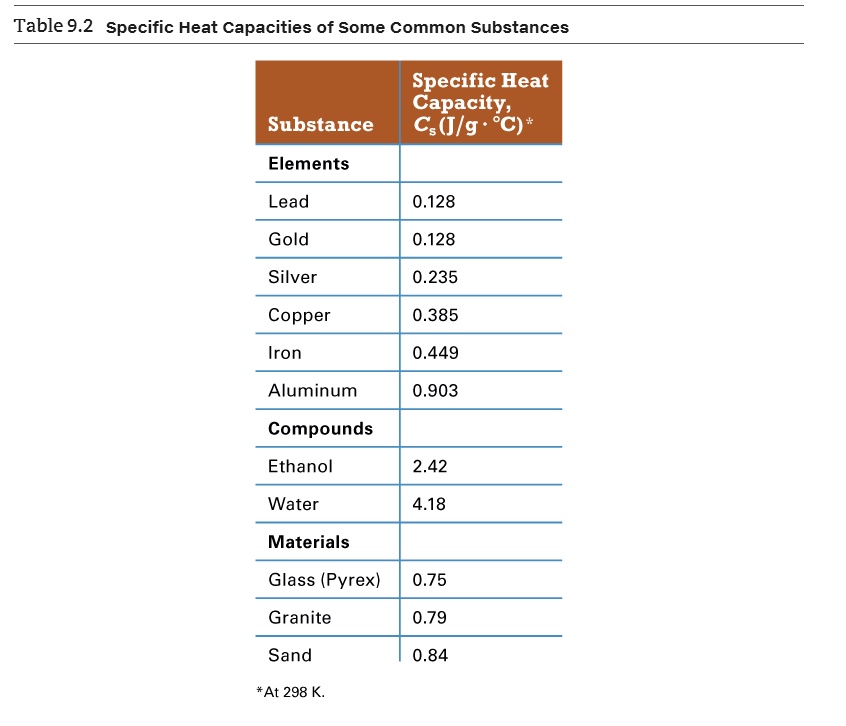
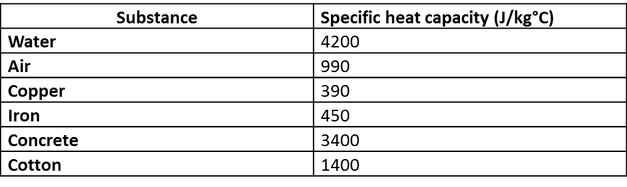
SOLVED: Table 9.2: Specific Heat Capacities of Some Common Substances Specific Heat Capacity; Cs (J/g *°C) Substance Elements Lead 0.128 Gold 0.128 Silver 0.235 Copper 0.385 Iron 0.449 Aluminum 0.903 Compounds Ethanol

Derived mean values of the specific heat of pure iron in comparison... | Download Scientific Diagram

The Heat Capacity and Thermodynamic Properties of the Iron Oxides and Their Relation to the Mineral Core of the Iron Storage Protein Ferritin | Semantic Scholar
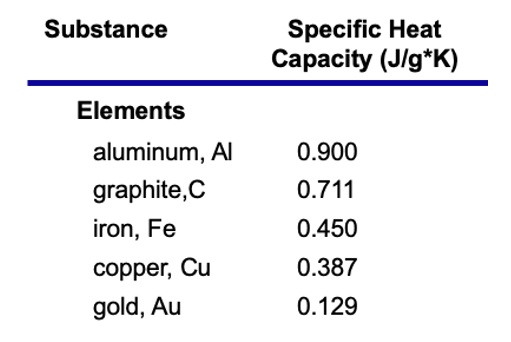
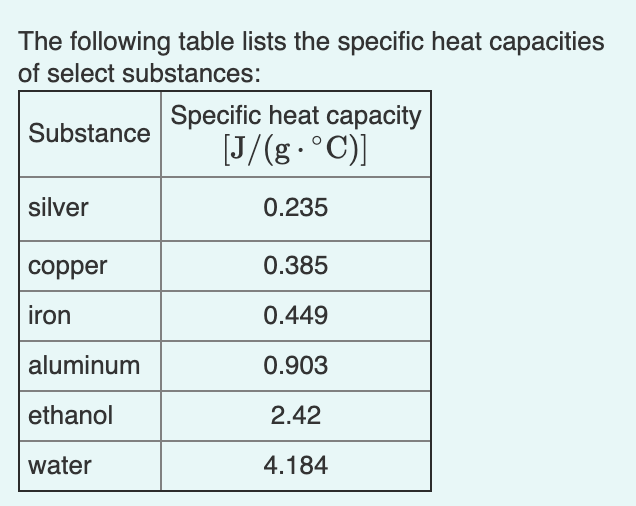
SOLVED: Text: Substance Specific Heat Capacity (J/g*K) Elements: aluminum, Al; graphite, C; iron, Fe; copper, Cu; gold, Au 0.900 J/g*K, 0.711 J/g*K, 0.450 J/g*K, 0.387 J/g*K, 0.129 J/g*K

Calculate the amount of heat required to raise the temperature of 5 g of iron from `25^(@)C \"to\" - YouTube

Comparison between the calculated and experimental heat capacity of... | Download Scientific Diagram

Calculate the energy required to heat 790.0g of iron from −2.6°C to 14.9°C. Assume the specific heat - brainly.com




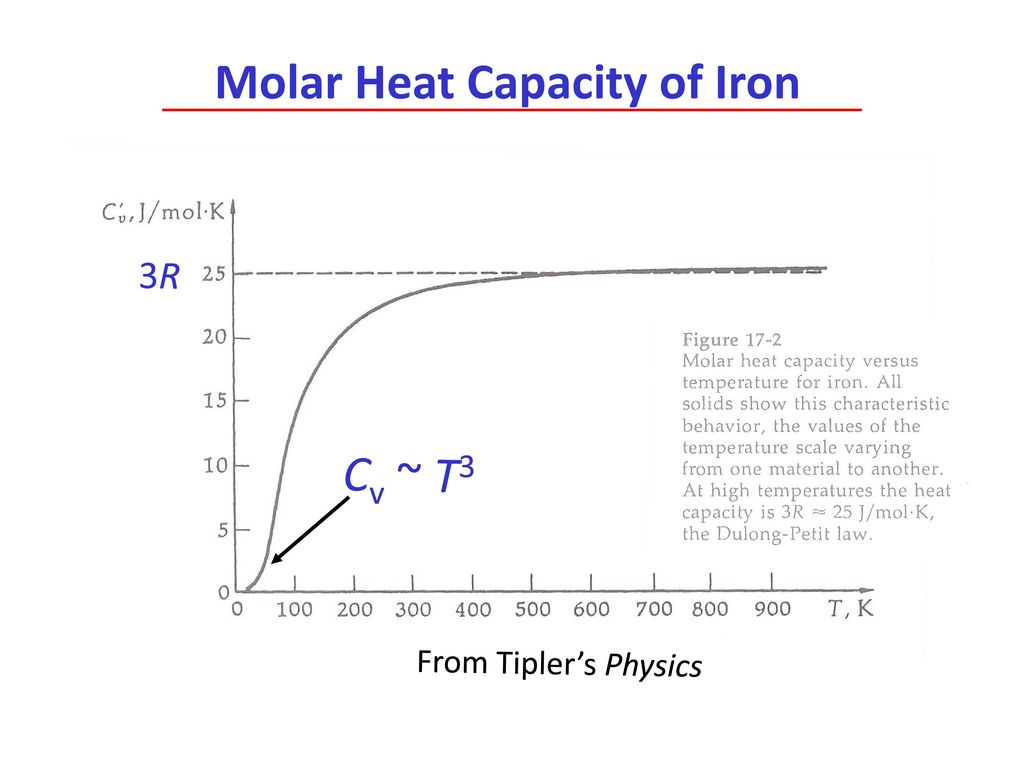
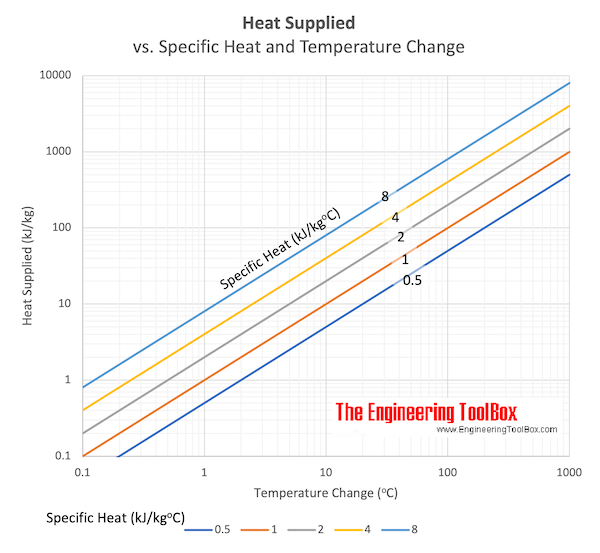

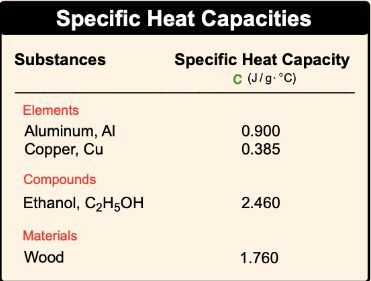

![Molar heat capacity of iron. Symbols indicate measurements from [10]. | Download Scientific Diagram Molar heat capacity of iron. Symbols indicate measurements from [10]. | Download Scientific Diagram](https://www.researchgate.net/publication/332219094/figure/fig1/AS:744154090987520@1554431569138/Molar-heat-capacity-of-iron-Symbols-indicate-measurements-from-10.png)